
Cheng Jin, Ph.D., Professor
Email: jinc@im.ac.cn
Telephone: 64807425
Dr. Cheng Jin has been focusing on investigation of the biosynthesis and function of microbial cell wall. His reearch fields include: i) biosynthesis and function of fungal cell wall glycoprotein and polysaccharide of fungal pathogens; ii) biosynthesis and function of extracellular polysaccharide of baterial pathogens; iii) Expression of glycoprotein in fungal cells; and iv) biosynthesis and function of surface glycans in archaea. More than 90 papers and 2 books were published.
Dr. Jin currently serves as Member of the Steering Committee of the Asian Consortium of Glycoscience and Glycotechnology, Associate Editor-in-Chief of Microbiology China, and members of Editorial Board of Chinese Journal of Biotechnology and Mycosystema.
Research Areas
Dr. Cheng Jin has been focusing on investigation of the biosynthesis and function of microbial cell wall. His reearch fields include: i) biosynthesis and function of fungal cell wall glycoprotein and polysaccharide of fungal pathogens; ii) biosynthesis and function of extracellular polysaccharide of baterial pathogens; iii) Expression of glycoprotein in fungal cells; and iv) biosynthesis and function of surface glycans in archaea. More than 90 papers and 2 books were published.
Education
M.S., 1990, Graduate School, Fudan University
Ph.D., 1993, Institute of Microbiology, CAS
Work Experience
1993.09—1995.12:Assistant Professor, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
1996.01—1997.04:Postdoc. Fellow, Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
1997.04—1999.10:Associate Professor, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
1999.11—2016.11:Professor, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
2016.12—2020.01:Professor, Guangxi Academy of Sciences, Nanning, Guangxi
2020.02—present: Professor, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China
Publications
- Shang, J., Qiu, R., Wang, J., Liu, J., Zhou, R., Yang, S., Zhang S., and Jin, C.*(1999)Molecular cloning and expression of Galb1,3GalNAc a2,3-sialyltransferase from human fetal liver. Eur. J. Biochem., 265: 580-588.
- Liu, N., Jin, C.*, Zhu, Z.*, Tao, H., and Zhang S.(1999)Stage-specific expression of a1,2-fucosyltransferase and a1,3-fucosyltransferase (FT) during mouse embryogenesis. Eur. J. Biochem., 265: 258-263.
- Xia, G., Han, X., Ding, H., Yang, S., Zhang, S., and Jin, C.*(2000)Cloning and overexpression of a cytidine 5’-monophosphate N-acetylneuraminic acid synthetase from Escherichia coli. J. Molecular Catalysis B: Enzymatic, 10: 201-208.
- Liu, J., Zhou, R., Zhang,N., Rui, J., and Jin, C. (2000) Biological function of a novel gene overexpressed in human hepatocellular carcinoma. Chinese Medical Journal, 113(10): 881-885.
- Xia, G., Jin, C., Zhou, J., Yang, S., Zhang, S., and Jin, C.* (2001) A novel chitinase having a unique mode of action from Aspergillus fumigatus YJ-407. Eur. J. Biochem., 268(14): 4079-4085.
- Hu, H., Wang, G., Yang, H., Zhou, J., Mo, L., Yang, K., Jin, C., Jin, C., and Rao, Z.* (2004) Crystallization and preliminary crystallographic analysis of a native chitinase from the fungal pathogen Aspergillus fumigatus YJ-407. Acta Crystallogr. D Biol. Crystallogr., 60(Pt5): 939-940.
- Liu, G., Jin, C., and Jin, C.* (2004) CMP-N-acetylneuraminic acid synthetase from E. coli K1 is a bi-functional enzyme: Identification of minimal catalytic domain for synthetase activity and novel functional domain for platelet-activating factor acetylhydrolase activity. J. Biol. Chem., 279(17): 17738-17749.
- Wang, D. P., Li, H. G., Li, Y. J., Guo, S. C., Yang, J., Qi, D. L., Jin, C.*, and Zhao, X. Q.* (2006) Hypoxia-inducible factor 1alpha cDNA cloning and its mRNA and protein tissue specific expression in domestic yak (Bos grunniens) from Qinghai-Tibetan plateau. Biochem. Biophys. Res. Commun., 348(1): 310-319.
- Li, H., Zhou, H., Luo, Y., Ouyang, H., Hu, H., and Jin, C.* (2007) Glycosylphosphatidylinositol (GPI)-anchor is required in Aspergillus fumigatus for morphogenesis and virulence. Mol. Microbiol., 64(4): 1014-1027.
- Zhuo, H., Hu, H., Zhang, L., Li, R., Ouyang, H., Ming, J., and Jin, C.* (2007) Protein O-mannosyltransferase 1 (AfPmt1p) in Aspergillus fumigatus is crucial for cell wall integrity and conidia morphology especially at an elevated temperature. Eukaryotic Cell, 6(12): 2260-2268.
- Li, Y., Zhang, L., Wang, D., Zhou, H., Ouyang, H., Ming, J., and Jin, C.* (2008) Deletion of the msdS gene induces abnormal polarity and septation in Aspergillus fumigatus. Microbiol., 154: 1960-1972.
- Jiang, H., Ouyang, H., Zhou, H., and Jin C.* (2008) GDP-mannose pyrophosphorylase is essential for cell wall integrity, morphogenesis and viability of Aspergillus fumigatus. Microbiol., 154: 2730-2739.
- Zhang, L., Zhou, H., Ouyang, H., Li, Y., and Jin, C.* (2008) Afcwh41 is required for cell wall synthesis, conidiation, and polarity in Aspergillus fumigatus. FEMS Microbiol. Lett., 289(2): 155-166.
- Li, Y., Fang, W., Zhang, L., Ouyang, H., Zhou, H., Luo, Y., and Jin, C.* (2009) Class IIC α-mannosidase AfAms1 is required for morphogenesis and cellular function in Aspergillus fumigatus. Glycobiol., 19 (6): 624-632.
- Zhang, L., Feng, D., Fang, W., Ouyang, H., Luo, Y., Du, T., and Jin, C.* (2009) Comparative proteomic analysis of an Aspergillus fumigatus mutant deficient in glucosidase I (AfCwh41). Microbiol, 155: 2157-2167.
- Lü, Y., Yang, H., Hu, H., Wang, Y., Rao, Z., and Jin, C.* (2009) Mutation of Trp137 to glutamate completely removes transglycosyl activity associated with the Aspergillus fumigatus AfChiB1. Glycoconjugate J., 26: 525-534.
- Fang, W., Yu, X., Wang, B., Zhou, H., Ouyang, H., Ming, J., and Jin, C.* (2009) Characterization of the Aspergillus fumigatus phosphomannose isomerase Pmi1 and its impact on cell wall synthesis and morphogenesis. Microbiol., 155: 3281-3293.
- Ouyang, H., Luo, Y., Zhang, L., Li, Y., and Jin, C.* (2010) Proteome analysis of Aspergillus fumigatus total membrane proteins identifies proteins associated with the glycoconjugates and cell wall biosynthesis using 2D LC-MS/MS. Mol. Biotechnol., 44: 177-189.
- Fang, W., Ding, W., Wang, B., Zhou, H., Ouyang, H., Ming, J., and Jin, C.* (2010) Reduced expression of the O-mannosyltransferase 2 (AfPmt2) leads to deficient cell wall and abnormal polarity in Aspergillus fumigatus. Glycobiology, 20(5): 542-552.
- Li, K., Ouyang, H., Lü, Y., Liang, J., Wilson, I. B. H., and Jin, C.* (2011) Repression of N-glycosylation triggers the unfolded protein response (UPR) and over-expression of cell wall protein and chitin in Aspergillus fumigatus. Microbiol., 157: 1968-1979.
- Song, L., Zhou, H., Cai, X., Li, C., Liang, J., and Jin, C.* (2011) NeuA O-acetylesterase activity is specific for CMP-activated O-acetyl sialic acid in Streptococcus suis serotype 2. Biochem. Biophys. Res. Commun., 410: 212-217.
- Yan, J., Du, T., Zhao, W., Hartmann, T., Lu, H., Lü, Y., Ouyang, H., Jiang, X., Sun, L., and Jin, C.* (2013) Transcriptome and biochemical analysis reveals that suppression of GPI-anchor synthesis leads to autophagy and possible necroptosis in Aspergillus fumigatus. PLoS ONE, 8(3): e59013. doi:10.1371/ journal.pone.0059013.
- Ouyang, H., Chen, X., Lü, Y., Wilson, I. B. H., Tang, G., Wang, A., and Jin, C.* (2013) One single basic amino acid at ω-1 or ω-2 site is a signal that retains glycosylphosphatidylinositol (GPI) anchored protein in plasma membrane of Aspergillus fumigatus. Eukaryotic Cell, 12(6): 889-899.
- Zhao, W., Lü, Y., Ouyang, H., Zhou, H., Yan, J., Du, T., and Jin, C.* (2013) N-glycosylation of Gel1 or Gel2 is vital for cell wall β-glucan synthesis in Aspergillus fumigatus. Glycobiology, 23 (8): 955-968.
- Fang, W.#, Du, T.#, Raimi, O. G., Hurtado-Guerrero, R., Urbaniak, M. D., Ibrahim, A. F. M., Ferguson, M. A. J., Jin, C.*, and van Aalten, D. M. F.* (2013) Genetic and structural validation of Aspergillus fumigatus UDP-N-acetylglucosamine pyrophosphorylase as an antifungal target. Mol. Mocrobiol., 89(3): 479-493.
- Fang, W.#, Du, T.#, Raimi, O. G., Hurtado-Guerrero, R., Mariño, K., Ibrahim, A. F. M., Albarbarawi, O., Ferguson, M. A. J., Jin, C.*, and van Aalten, D. M. F.* (2013) Genetic and structural validation of Aspergillus fumigatus N-acetylphosphoglucosamine mutase as an antifungal target. Bioscience Reports, 33(5): art: e00063. doi: 10.1042/BSR20130053. (4.059, Q3)
- Chen, F., Tao, Y., Jin, C., Xu, Y., Lin, B.-X.* (2015) Enhanced production of polysialic acid by metabolic engineering of Escherichia coli. Appl. Microbiol. Biotechnol., DOI 10.1007/s00253-015-6391-x.
- Wang, J., Zhou, H., Lua, H., Du, T., Luo, Y., Wilson, I. B.H., Jin, C.* (2015) Kexin-like endoprotease KexB is required for N-glycan processing, morphogenesis and virulence in Aspergillus fumigatus. Fungal Genetics and Biology, 76: 57-69.
- Lu, H., Lü, Y., Ren, J., Wang, Z.,Wang, Q., Luo, Y., Han, J., Xiang, H., Du., Y., Jin, C.* (2015) Identification of the S-layer glycoproteins and their covalently linked glycans in the halophilic archaeon Haloarcula hispanica. Glycobiology, 25(11): 1150-1162.
- Yang, J.*, Zhou, Y., Zhang, L., Shah, N., Jin, C., Palmer, R. J., Jr., Cisar, J. O. (2016) Cell surface glycoside hydrolases of Streptococcus gordonii promote growth in saliva. Appl. Environ. Microbiol., 82:5278-5286.
- Sharma, P.G., Ouyang, H., Wang, Q., Luo, Y., Shi, B., Yang, J., Lu, Y., and Jin, C.* (2016) Insight into enzymatic degradation of corn, wheat, and soybean cell wall cellulose using quantitative secretome analysis of Aspergillus fumigatus. J. Proteome Res., 15 (12): 4387–4402. DOI: 10.1021/acs.jproteome.6b00465.
- Lü, Y.*, Lu, H., Wang, S., Han, J., Xiang, H., and Jin, C.* (2017) An acidic exopolysaccharide from Haloarcula hispanica ATCC33960 and two genes responsible for its synthesis. Archaea, 2017: 5842958, 1-12 (DOI: 10.1155/2017/5842958).
- Geno, K. A., Bush, C. A., Wang, M., Jin, C., Nahm, M. H.*, and Yang, J.* (2017) WciG O-acetyl transferase functionality differentiates pneumococcal serotypes 35C and 42. J. Clinical Microbiol., DOI: 10.1128/JCM.00822-17.
- Lü, Y.#, Liu, W.#, Liang, H., Zhao, S., Zhang, W., Liu, J., Jin, C.*, and Hu, H.* (2018) NDM-1 encoded by a pNDM-HN380-Like Plasmid pNDM-BJ03 in clinical Enterobacter cloacae. Diagnostic Microbiology & Infectious Disease, 90 (2): 153-155.
- Ouyang, H., Du, T., Zhou, H., Wilson, I. B. H., Yang, J., Latgé, J.-P., Jin, C.* (2019) Aspergillus fumigatus phosphoethanolamine transferase gene gpi7 is required for proper transportation of the cell wall GPI-anchored proteins and polarized growth. Scientific Reports, 9: 5857. DOI: https://doi.org/10.1038/s41598-019-42344-1.
- Du, T., Ouyang, H., Voglmeir, J., Wilson, I. B. H., Jin, C.* (2019) Aspergillus fumigatus Mnn9 is responsible for mannan synthesis and required for covalent linkage of mannoprotein to the cell wall. Fungal Genetics and Biology, 128: 20-28. DOI: https://doi.org/10.1016/ j.fgb.2019.03.006.
- Xie, M., Zhao, X., Lü, Y., Jin, C.* (2019) Chitin deacetylases Cod4 and Cod7 are involved in polar growth of Aspergillus fumigatus. MicrobiologyOpen. 2019: 00: e943. https://doi.org /10.1002/mbo3.943.
- Li, L., Ren, M., Xu, Y., Jin, C., Zhang, W., Dong, X.* (2019) Enhanced glycosylation of an S-layer protein enables psychrophilic methanogenic archaeon to adapt to elevated temperatures in abundant substrates. FEBS Lett., 594(2020): 665–677. doi: 10.1002/1873-3468.13650.
- Xu, Y., Zhou, H., Zhao, G., Yang, J., Luo, Y., Sun, S., Wang, Z., Li, S., Jin, C.* (2020) Genetical and O-glycoproteomic analyses reveal the differential functions of three protein O-mannosyltransferases in phytopathogen Fusarium oxysporum f.sp. cucumerinum. Fungal Genetics and Biology, 134: 103285. https://doi.org/10.1016/j.fgb.2019.103285.
- Ndubuisi, A. I., Qin, Q., Liao, G., Wang, B., Moneke N. A., Ogbonna C. J., Jin, C.*, Fang, W.* (2020) Effects of various inhibitory substances and immobilization on ethanol production efficiency of a thermotolerant Pichia kudriavzevii. Biotechnol Biofuels, 13: 91. https://doi.org/10.1186/s13068-020-01729-5.
- Lu, H.#*, Pei, C.#, Zhou, H., Lü, Y., He, Y., Li, Y., Han, J., Xiang, H., Eichler, J, Jin, C.* (2020) Agl22 and Agl23 are involved in the synthesis and utilization of the lipid-linked intermediates in the glycosylation pathways of the halophilic archaeaon Haloarcula hispanica. Molecular Microbiology, 114: 762–774. http://dx.doi.org/10.1111/mmi.14577.
- Zhao, G., Xu, Y., Ouyang, H., Luo, Y., Sun, S., Wang, Z., Yang, J., Jin, C.* (2020) Protein O-mannosylation affects protein secretion, cell wall integrity, and morphogenesis in Trichoderma reesei. Fungal Genetics and Biology, 144: 103440. https://doi.org/10.1016/ j.fgb.2020.103440.
- Zhou, H., Xu, Y., Ebel, F., Jin, C.* (2021) Galactofuranose (Galf)-containing sugar chain is essential for the growth, conidiation and virulence of F. oxysporum f.sp. cucumerinum. PLoS ONE 16(7): e0250064. https://doi.org/10.1371/journal.pone.0250064.
- Sharma, G. P.#, Ouyang, H.#, Zhao, G., Xie, M., Zhou, H., Yang, J., Jin, C.* (2021) Altered N-glycan processing in Trichoderma reesei affects the morphogenesis and improves the degradation of lignocellulose. Microbiology China, 48(10): 3432−3448.
- Gao, L., Ouyang, H., Pei, C., Zhou, H., Yang, J.* and Jin, C.* (2022) Emp47 and Vip36 are required for polarized growth and protein trafficking between ER and Golgi apparatus in opportunistic fungal pathogen Aspergillus fumigatus. Fungal Genetics and Biology, 158: 103638. https://doi.org/10.1016/j.fgb.2021.103638.
- Yang, J.#*, Ma, W.#, Wu, Y.#, Zhou, H., Song, S., Cao, Y., Wang, C., Liu, X., Ren, J., Duan, J., Pei, Z., Jin, C.* (2021) O-Acetylation of capsular polysialic acid enables Escherichia coli K1 escaping from Siglec-mediated innate immunity and lysosomal degradation of E. coli-containing vacuoles in Macrophage-like cells. Microbiology Spectrum, 9(3): e00399-21. https://doi.org/10.1128/ spectrum.00399-21.
- Ouyang, H.#*, Zhang, Y#, Zhou, H., Ma, Y., Li, R., Yang, J., Wang, X.*, Jin, C.* (2021) Deficiency of GPI glycan modification by ethanolamine phosphate results in increased adhesion and immune resistance of Aspergillus fumigatus. Frontiers in Cellular and Infection Microbiology, 11:780959. doi: 10.3389/fcimb.2021.780959.
- Zhou, Y.#, Du, C.#, Odiba, A.S., He, R., Ahamefule, C. S., Wang, B., Jin, C.* and Fang, W.* (2021) Phosphoglucose isomerase plays a key role in sugar homeostasis, stress response and pathogenicity in Aspergillus flavus. Frontiers in Cellular and Infection Microbiology, 11:777266. doi: 10.3389/fcimb.2021.777266.
- Singh, P.#, Xie, J.#, Qi, Y., Qin, Q., Jin, C., Wang, B.*, Fang, W.* (2021) A thermotolerant marine Bacillus amyloliquefaciens S185 producing iturin A5 for antifungal activity against Fusarium oxysporum f. sp. cubense. Marine. Drugs, 19: 516. https://doi.org/10.3390/ md19090516.
- Chakraborty, A.#, Fernando, L. D.#, Fang, W.#, Widanage, M. C. D.#, Wei, P., Jin, C., Fontaine, T., Latgé, J.-P.*, Wang, T.* (2021) A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR. Nature Communications, 12: 6346. https://doi.org/10.1038/s41467-021-26749-z.
- Gao, L., Ouyang, H., Pei, C., Zhou, H., Yang, J.* and Jin, C.* (2022) Emp47 and Vip36 are required for polarized growth and protein trafficking between ER and Golgi apparatus in opportunistic fungal pathogen Aspergillus fumigatus. Fungal Genetics and Biology, 158: 103638. https://doi.org/10.1016/j.fgb.2021.103638.
- Xu, Y.#, Li, Y.#, You, X.#, Pei, C.#, Wang, Z. A., Jiao, S., Zhao, X., Lin, X., Lv, Y., Jin, C., Gao, G. F., Li J.-J.*, Wang, Q.*, Du, Y.* (2022) Novel insight into the sulphated glucuronic acid-based anti-SARS-CoV-2 mechanism of exopolysaccharides from halophilic archaeaon Haloarcula hispanica. Front. Chem., 10, 871509. DOI: 10.3389/fchem.2022.871509.
- Odiba, A. S., Ezechukwu, C. S., Liao, G., Li, S., Chen, Z., Liu, X., Fang, W., Jin, C., Wang, B.* (2022) Loss of NSE-4 perturbs genome stability and DNA repair in Caenorhabditis elegans. International Journal of Molecular Sciences, 23, 7202. https://doi.org/10.3390/ijms23137202.
- Zhou, Y.#, Yan, K.#, Qin, Q., Raimi, O., Du, C., Wang, B., Ahamefule, C. S., Kowalski, B., Jin, C., van Aalten, D. M. F.*, and Fang, W.* (2022) Phosphoglucose isomerase is essential for Aspergillus fumigatus cell wall biogenesis. mBio, e01426-22. https://doi.org/10.1128/mbio.01426-22.
- Usman, S., Du, C., Qin, Q., Odiba, A. S., He, R., Wang, B., Jin, C.*, Fang, W* (2022) Phosphomannose isomerase is involved in development, stress responses and pathogenicity of Aspergillus flavus. Microbiology Spectrum, https://doi.org/10.1128/spectrum.02027-22.
Pei, C.#, Lu, H.#, Ma, J., Eichler, J., Guan, Z., Gao, L., Liu, L., Zhou, H., Yang, J.*, Jin, C.* (2023) AepG is a dolichol phosphate glucuronosyltransferase involved in acidic exopolysaccharide synthesis and contributes to environmental adaptation of Haloarcula hispanica. J. Biol. Chem., 299(2): 102911. doi:10.1016/j.jbc.2023.102911.
Okoro, N. O., Odiba, A. S., Yu, Q., He, B., Liao, G., Jin, C., Fang, W.*, Wang, B.* (2023) Polysaccharides extracted from Dendrobium officinale grown in different environments elicit varying health benefits in Caenorhabditis elegans. Nutrients, 15: 2641.
Odiba, A.S., Liao, G., Ezechukwu, C.S., Zhang, L., Hong, Y., Fang, W., Jin, C., Gartner, A., Wang, B.* (2023) Caenorhabditis elegans NSE3 homolog (MAGE-1) is involved in genome stability and acts in inter-sister recombination during meiosis. Genetics, 225(2): iyad149. Doi: 10.1093/genetics/iyad149.
Usman, Sayed, Ge, X., Xu, Y., Qin, Q., Xie, J., Wang, B., Jin, C.*, Fang, W.* (2023) Loss of phosphomannose isomerase impairs growth, perturbs cell wall integrity, and reduces virulence of Fusarium oxysporum f. sp. cubense on banana plants. J. Fungi, 9(4): 478. Doi: 10.3390/jof9040478.
Xie, J.#, Singh, P,#, Qi, Y., Singh, R. K., Qin, Q., Jin, C., Wang, B.*, Fang, W.* (2023) Pseudomonas aeruginosa strain 91: A multifaceted biocontrol agent against banana Fusarium wilt. J. Fungi, 9: 1047. https://doi.org/10.3390/jof9111047.
He, R., Wei, P., Odiba, A. S., Gao, L., Usman, S., Gong, X., Wang, B., Wang, L., Jin, C., Lu, G., Fang, W.* (2024) Amino sugars influence Aspergillus fumigatus cell wall polysaccharide biosynthesis, and biofilm formation through interfering galactosaminogalactan deacetylation. Carbohydrate Polymers, 324: 121511.
Qi, Y., Qin, Q., Liao, G., Tong, L., Jin, C., Wang, B.*, Fang, W.* (2024) Unveiling the super tolerance of Candida nivariensis to oxidative stress: insights into the involvement of a catalase. Microbiology Spectrum, 12(2): e03169-23. DOI: 10.1128/spectrum.03169-23.
Xu, Y., Qi, Y., Xie, J., Qin, Q., Huang, G., Tang, P., Jin, C., Wang, B.*, Li, Y.*, Fang, W.* (2024) A Yokenella regensburgei strain effectively removes Cu(II), Pb(II), and Cd(II) through a combination of absorption and mineralization modes. Journal of Water Process Engineering, 58: 104805.
(# First author;* Corresponding author)
Patents
( 1 ) 一株荚膜多聚唾液酸高度O-乙酰化修饰的大肠杆菌及其应用, 发明专利, 2021, 第 3 作者, 专利号: CN112375707A
( 2 ) 一种多糖及其制备方法, 专利授权, 2020, 第 2 作者, 专利号: CN107034254B
( 3 ) 基因HAH_1662在制备多糖中的应用, 专利授权, 2020, 第 2 作者, 专利号: CN107163110B
( 4 ) 基因HAH_1667在制备多糖中的应用, 专利授权, 2019, 第 2 作者, 专利号: CN107056900B
( 5 ) 一种产唾液酸乳糖大肠杆菌工程菌株的构建方法, 发明专利, 2018, 第 4 作者, 专利号: CN107904253A
( 6 ) 一种曲霉表达载体及其应用, 发明专利, 2010, 第 1 作者, 专利号: CN101760471A
( 7 ) 将蛋白定位于细胞膜的多肽及其应用, 发明专利, 2010, 第 1 作者, 专利号: CN101759783A
( 8 ) 将蛋白定位于细胞壁的多肽及其应用, 发明专利, 2010, 第 1 作者, 专利号: CN101759784A
( 9 ) 将蛋白定位于细胞膜和/或细胞壁的多肽及其应用, 发明专利, 2010, 第 1 作者, 专利号: CN101759782A
( 10 ) 由烟曲霉产生的一种新的几丁质酶, 发明专利, 2000, 第 1 作者, 专利号: CN1269405
Conferences
2. 金城 CMP-唾液酸合成酶基因的改造及其在大肠杆菌中的高效表达. 第三次全国酶工程学术讨论会, 2000年8月2-5日, 成都.
3. Jin, C. A novel chitinase with chitin-1,4-β-chitobiosidase and endochitinase activities from Aspergillus fumigatus. The 6th Japan-China Joint Symposium on Enzyme Engineering, Oral presentation, Oct.15-18, 2000, Kyoto, Japan.
4. 金城. 糖链代谢酶的研究及其发展趋势. 酶工程研究前沿研讨会, 2001年5月28日-6月4日, 张家界.
5. Jin, C. and Jin, C. Minimal functional domain of cytidine 5’-monophosphate N-acetylneuraminic acid (CMP-NeuAc) synthetase from E. coli. The 7th China-Japan Joint Symposium on Enzyme Engineering, Oral presentation, Sept.21-26, 2002, Xian, China.
6. Jin, C. Elevated expression of Galβ1,3GalNAc a2,3-sialyltransferase and altered expression of glycans on hepatocyte cell surface activated by MHBst/HBx of hepatitis B virus. The 192nd Xiangshan Science Conference on “Prospects in structure and function of sugar chains”, Oct. 9-11, 2002, Gragrant Hill Hotel, Beijing, China.
7. 金城. 微生物糖链代谢酶的研究. 第四届全国酶工程学术交流讨论会, 大会报告, 2003年10月12-17日, 无锡.
8. Jin, C. Glycosylation in Aspergillus fumigatus. The 8th China-Japan-Korea Joint Symposium on Enzyme Engineering, Keynote Lecture, Oct.24-26, 2004, Hangzhou, China.
9. 金城. 微生物的糖基化与功能. 资源生物技术与糖工程学术研讨会, 大会报告, 2005年7月7-9日, 济南, 中国.
10. 金城. 微生物糖组学研究. 中国生物工程学会年会, 大会报告, 2005年7月16-18日, 天津, 中国.
11. Jin, C. Glycosylation pathway in Aspergillus fumigatus. Enzyme Engineering XVIII, invited speaker, Oct.9-14, 2005, Gyeong-ju, Korea.
12. 金城. 烟曲霉糖蛋白的糖基化及功能. 第五届中国酶工程学术研讨会, 大会报告, 2005年 11月15-20日, 海口, 中国.
13. 金城. 丝状真菌甘露糖基化生物合成途径及其生物学功能. 第一届全国糖生物学会议, 大会报告, 2006年8月20-22日, 大连, 中国.
14. Yang, X., Yang S., Han B., and Jin C. Structure and function of β-Glycosidase from Thermus nonproteolyticus. The 9th Japan-China-Korea Joint Symposium on Enzyme Engineering, Oral presentation, Oct.30-Nov.2, 2006, Otsu, Japan.
15. 金城. 烟曲霉(Aspergillus fumigatus) 几丁质酶Chi44的结构与功能. 中国甲壳素及其衍生物专家研讨会, 大会报告, 2007年6月2-3日, 青岛, 中国.
16. 金城. 丝状真菌蛋白质糖基化及其生物学功能. 第二届资源生物技术与糖工程学术研讨会, 大会报告, 2007年7月14-17日, 威海, 中国.
17. 金城. 丝状真菌甘露糖基化生物合成途径及其生物学功能. 2007年微生物学会学术年会, 大会报告, 2007年8月12-16日, 乌鲁木齐, 中国.
18. Jin, C. Mutation of Trp137 to glutamate completely removes transglycosyl activity associated with the Aspergillus fumigatus AfChiB1. The 10th Korea-China-Japan Joint Symposium on Enzyme Engineering, Nov. 2-5, 2008, Bushan, Korea.
19. Jin, C. Glycosidase and glycosyltransferase in fungus Aspergillus fumigatus. Commemorative Symposium on Enzyme Engineering --- the 30th Anniversary of Japanese Society of Enzyme Engineering, Invited lecture, Nov. 13-15, 2008, Kisaradu, Japan.
20. Jin, C. Glycosidase and glycosyltransferase in fungus Aspergillus fumigatus. ISHAM 2009 Beijing Satellite Symposia, Invited lecture, May 29-31, 2009, Beijing, China.
21. Jin, C. Glycosylation is required for cell wall biogenesis and morphogenesis in opportunistic fungus Aspergillus fumigatus. the 1st Communications of Glycobiology and Glycotechnology, Invited lecture, Oct. 28-Nov.1, 2009, Tsukuba, Japan.
22. Jin, C. Functional analysis of glycosylation in filamentous fungus Aspergillus fumigatus. 第七屆海峽兩岸真菌學學術研討會, Nov.13-22, 2009, 台湾。
23. Jin, C. Bi-functional NeuA protein from Streptocossus suis. 2009 Sino-German Symposium on Streptococcus Infectious Diseases and Beyond, Invited lecture, Dec. 2-3, 2009, Guangzhou, China.
24. 金城. 烟曲霉的发病机制——毒力因子与抗真菌药物靶点. 首届曲霉与曲霉病新进展高峰论坛. 特邀报告,2010年7月16-18日,成都.
25. 金城. 真菌糖生物学——揭示糖链复杂功能的突破口?2010年全国糖生物学学术会议, 大会报告, 2010年8月10-13日,长春.
26. 金城. 蛋白质糖基化修饰的功能研究. 2010年北京生物化学与分子生物学会年会, 大会报告, 2010年12月4日,北京.
27. Cheng Jin. Glycosylation and its function in the filamentous fungus Aspergillus fumigatus. Invited lecture in Department of Chemistry, University of Natural Resources and Life Sciences, Feb.14, 2011, Vienna, Austria.
28. Jin, C. Synthesis and regulation of capsular sialic acid in E. coli and Streptococcus suis serotype 2. The third Asian Conference of Glycobiology and Glycotechnology, Invited lecture, Oct. 27-29, 2011.
29. Jin, C. Glycosylation and its function in Aspergillus fumigatus. 2011 Annual Conference of the Society for Glycobiology, invited lecture, Nov.12, 2011, Seattle, USA.
30. Jin, C. Synthesis and regulation of capsular sialic acid in E. coli and Streptococcus suis. The 12th Japan-China-Korea Joint Symposium on Enzyme Engineering, ERATO invited lecture, May 28–June 1, 2012, Kanazawa, Japan.
31. Jin, C. Transcriptome and biochemical analysis reveals that suppression of GPI-anchor synthesis leads to autophagy and necroptosis in Aspergillus fumigatus. The 22nd International Symposium on Glycoconjugate (GLYCO22), invited keynote lecture, June 23-28, 2013, Dalian, China.
32. Jin, C. Genetic and structural validation of enyzmes required for activation of UDP-GlcNAc in Aspergillus fumigatus as potential antifungal targets. The Fifth Asian Conference of Glycobiology and Glycotechnology, Invited lecture, Oct. 16-18, 2013, Khon Khae, Thailand.
33. Jin, C. Genetic and structural validation of enzymes required for activation of UDP-GlcNAc in Aspergillus fumigatus as potential antifungal targets. International Symposium on Biocatalysis and Biosynthetic Engineering, Invited lecture, Nov. 11-13, 2013, Shanghai, China.
34. 金城. 嗜盐古菌西班牙盐盒菌表层蛋白糖基化修饰. 2016年全国糖生物学会, 大会报告,2016年8月5-7日, 江苏南通.
35. Jin, C., Lu, H., Lu, Y., Ren, J., Wang, Z., Wang, Q., Luo, Y., Han, J., Xiang, H., Du, Y., Yang, J. Glycosylation of the S-layer protein in Haloarcula hispanica. 8th ACGG Annual Conference, Invited lecture, Dec. 17-20, 2017, Hongkong.
36. Jin, C., Xie, M., Lü, Y., Zhao, X., Su, J., Chen, H., Yang, J. Biochemical and genetic analyses of cod4 and cod7 gene reveal that chitin deacetylation is involved in polar growth in Aspergillus fumigatus. The 15th Japan-China-Korea Joint Symposium on Enzyme Engineering, Oral presentation, June30-July 2, 2018, Kyoto, Japan.
37. Jin, C. Glycoproteins and polysaccharides in microbial pathogens. 2018年第四屆海峽兩岸生命科學論壇,大会报告,August 27, 2018,台北。
38. 金城. 烟曲霉几丁质脱乙酰酶Cod4和Cod7的功能研究. 2018年全国糖生物学会议, 大会报告,2018年9月22-23日,上海。
39. 金城. Function of glycosylphosphatidylinostiol (GPI) anchoring in protein sorting and cell wall synthesis in Aspergillus fumigatus. 2018年真菌人类病原高峰论坛, 大会报告,2018年11月2日, 北京。
40. 金城,许跃强,周 慧,赵光亚,杨静华,罗元明,孙树涛,王仲孚,李少杰. 尖孢镰刀菌O-甘露糖基化修饰的研究. 2019年全国糖生物工程会议,大会报告,2019年5月11日,青岛。
41. Cheng Jin. Cell wall mannan synthesis and its function in Aspergillus fumigatus. 1st International Symposium on Polysaccharide. Dec.4-5, 2019, Zhuhai.
42. Jin, C. Function of glycosylphosphatidylinostiol (GPI) anchoring in protein sorting and cell wall synthesis in Aspergillus fumigatus. 11th International Symposium on Glycosyltransferase, Invited lecture, June 19-23, 2018, Qingdao, China.
43. Du, T., Ouyang, H., Voglmeir, J., Wilson B.H. I., Jin, C. Cell wall mannan synthesis and its function in Aspergillus fumigatus. The first International Symposium on Polysaccharide, Invited lecture, Dec. 5-6, 2019, Zhuhai, China.
44. 金城 糖合成生物学与重要现代化。糖科学与大湾区重要产业发展论坛,大会报告,2023年3月24-26日,珠海横琴。
45. 金城 微生物糖组学与合成糖生物学。2022 年全国糖生物学会议,大会报告,2023年5月22-24日,武汉。
Students
已指导学生
张蕾 博士研究生 071010-生物化学与分子生物学
胡红焱 博士研究生 071010-生物化学与分子生物学
刘广超 博士研究生 071010-生物化学与分子生物学
李雁洁 博士研究生 071010-生物化学与分子生物学
李红 博士研究生 071010-生物化学与分子生物学
蒋鹤春 博士研究生 071010-生物化学与分子生物学
向四海 硕士研究生 071010-生物化学与分子生物学
王颖 硕士研究生 071010-生物化学与分子生物学
陈晓敏 硕士研究生 071010-生物化学与分子生物学
欧阳浩淼 博士研究生 071010-生物化学与分子生物学
吕洋 博士研究生 071010-生物化学与分子生物学
房文霞 博士研究生 071010-生物化学与分子生物学
宋丽丽 博士研究生 071010-生物化学与分子生物学
李开 博士研究生 071010-生物化学与分子生物学
丁卫平 硕士研究生 071010-生物化学与分子生物学
阎江洪 博士研究生 071010-生物化学与分子生物学
苏建臣 硕士研究生 071010-生物化学与分子生物学
杜婷 博士研究生 071010-生物化学与分子生物学
谢贻 硕士研究生 071010-生物化学与分子生物学
赵婉 博士研究生 071010-生物化学与分子生物学
王婧阳 博士研究生 071010-生物化学与分子生物学
卢化 博士研究生 071010-生物化学与分子生物学
代剑波 博士研究生 071010-生物化学与分子生物学
解明明 博士研究生 071010-生物化学与分子生物学
马玮 硕士研究生 071010-生物化学与分子生物学
高琳璐 博士研究生 071010-生物化学与分子生物学
王梦楠 硕士研究生 085238-生物工程
赵光亚 博士研究生 071010-生物化学与分子生物学
张秀梅 硕士研究生 085238-生物工程
裴彩霞 硕士研究生 085238-生物工程
许跃强 博士研究生 071010-生物化学与分子生物学
现指导学生
郝金斌 博士研究生 071010-生物化学与分子生物学
马加胤 博士研究生 071010-生物化学与分子生物学
Honors & Distinctions
Research Interests and advances
Our lab has been focusing on biosynthesis and functions of microbial cell wall glycoproteins and polysaccharides for over 30 years. Our research interests include investigation of synthesis of the surface polysaccharides and glycoproteins in fungal and bacterial pathogens, their functions during infection, and identification of new targets for diagnosis and therapies. We also interested in the post-translation modification of proteins in fungi and construction of expression system for glycoproteins. The major advances achieved in the structures, biosythetic pathways and functions of cell wall of fungal pathogens (Aspergillus fumigatus and Fusarium oxysporum), extracellular polysaccharides of bacterial pathogens (E. coli, Streptococcus suis, and Strptococcus pneumonae), and archaea (Haloarcula hispanica) are briefly summarized as the following.
1. Biosythesis of fungal cell wall and its function during infection
The cell wall is a unique structure of fungal cells, which is not only a physical barrier to proctect fungal cells in environment, but also a cellular structure to directly contact host cells. As cell wall does not exist in mammalians, it has been long taken as an ideal target for development of anti-fungal drugs and diagnosis as well.
1.1 Biosynthesis of cell wall and its function in infection caused by A. fumigatus
A. fumigatus is a major fungal pathogen causing invasive aspergillosis (IA). The mortality of IA is around 85%. The mortality still remains high at ~50% even treated with currently available drugs. The higher mortality is due to late diagnosis and limited drugs. There is an urgent need to develop new theurapies. To encourage the drug development, the U.S. Food and Drug Administration's (FDA's) issued the Generating Antibiotics Incentives Now (GAIN) Act to grants new antifungals “orphan status”, lower clinical trial barriers, and allow a 5-year extension of market exclusivity for anti-fungal drugs.
We have determined the structures of N- and O-glycans on glycoproteins produced by A. fumigatus. Through bio-informatical and proteomic analyses, the glycosylation pathways (N-glycosylation, O-glycosylation and GPI-anchoring) have been identified. Based on this information, the synthesis and functions of the presursors of cell wall glycoproteins and polysaccharides were investigated by genetical and biochemical methods. Our results show that pmi1, srb1, uap1, and ugm1 genes, which are responsible for activation of mannose (Man) and N-acetylglucosamine (GlcNAc) to produce GDP-Man and UDP-GlcNAc respectively, are essential for the synthesis of cell wall glycoproteins and polysaccharides. Deletion each of these genes causes phenotypes such as cell wall defect, cell death, abnormal morphogenesis, and porlar abnormality, suggesting that these genes are potential targets for anti-fungal drugs. The stt3, cwh41, msdS, and ams1 genes, which are invovlved in N-glycosylation and de-N-glycosylation, were found to be required for cell wall synthesis and polarized growth. The mechanisms have been elucidated. Investigation of the pmt1, pmt2 and pmt4 genes has shown that O-glycosylation is required for cell wall synthesis, thermotolerance, and polarity. Deletion each of these genes leads to different phenotypes, which has been confirmed due to different substrate specificities of each Pmt.
GPI anchoring is also involved in cell wall synthesis. Our results show that blocking of GPI anchoring leads to cell death, cell wall defect, and attenuated virulence of A. fumigatus. We further show that deficient GPI anchoring causes accumulation of proteins and PI in ER, which activates the ER-stress and PI3K, then triggers the release of ER-calcium, and eventually induces autophage and necroptosis. These results indicate that GPI anchoring is an essential post-translation modification for cell wall synthesis and virulence of A. fumigatus.
Recently, we have confirmed that Gel1, Gel2, Gel4, and Ecm33 are GPI-anchored membrane proteins. Glucanosyltransferases Gel1 and Gel2 require N-glycosylation for their proper folding. Mp1 has been confirmed as a specific GPI-anchored cell wall glycoprotein. Mnn9 is involved in the cross-link of Mp1 to cell wall glucan. Further study shows that maturation, sorting and localization of Mp1 are modulated by GPI-glycan modification. Interferring the modification of GPI-glycan leads to mis-localization of Mp1, cell wall defect, abnormal polarity, autophage, increased adherence, and stronger immune response in mouse model and innate immune cells. Our results demonstrate that GPI anchoring is essential for sorting and localization of cell wall proteins, which then affects the structure of cell wall, virulence and immune response of A. fumigatus (Figure 1).

Figure 1. Model of the cell wall synthesis in A. fumigatus. In A. fumigatus, glucanosyltransferases Gel1 and Gel2, and cell wall glycoprotein Mp1 are GPI-anchored proteins. (1) N-glycosylation is required for proper folding of Gel1 and Gel2. Properly folded Gel1 and Gel2 will be modified by GPI and transported to plasmic membrane to synthesize cell wall glucans. (2) During synthesis of GPI anchor, the last step involves in the modification of Man2 residue in GPI-glycan with phosphoethanolamine (EtNP) by Gpi7. Upon modification of Man2 residue with EtNP, GPI anchor becomes mature form and transferred to protein. Then GPI-anchored proteins will undergo a release of EtNP group on Man2 residue by Ted1, which allows the specific recognition of GPI-anchored proteins by p24 and the recuitment of GPI-abchored proteins into COP II vesicle. Once the modification of Man2 residue was inerferred, Mp1 is unable to cross-link to cell wall glucans. (3) After GPI-anchored proteins are transported into Golgi via COP II vesicle, glucanosyltransferases Gel1 and Gel2 are specifically expressed on plasmic membrane as the first or second residue at the C-terminal of protein is basic amino acid. (4) The cell wall glycoprotein Mp1 will be further processed by Mnn9, which initiates the elongation of O-linked glycan to form mannan. After release from plasmic membrane, Mp1 enters cell wall and cross-links to glucans. (5) In ER, lectin Emp47 is responsible for the sorting of N-glycosylated secreted proteins into COP II vesicle, which ensures proper transport of secreted glycoproteins into Golgi. (6) In Golgi, lectin Vip36 is responsible for the sorting of chaperons leaked from ER into COP I vesicle, which ensures protein retrieval from Golgi to ER and maintains ER-hemeostasis.
1.2 Contribution of cell wall glycoproteins and polysaccharides to virulence of Fusarium oxysporum
Fusarium oxysporum is a fungal pathogen causing wilts of various agriculural plants. Our research has identified that Pmt1, Pmt2 and Pmt4 are responsible for O-glycosylation in F. oxysporum. Although their functions are different, all of them contribute to virulence. The ugmA, ugmB and gfsA genes are responsible for synthesis of galactofuranose-containing sugar chains in cell wall and essential for virulence of F. oxysporum. As galactofuranose-containing sugar chain is not found in plant, the pmts, ugmA, ugmB and gfsA genes are ideal targets for anti-wilts.
2. Biosynthesis and function of extracelluar polysaccharides of bacterial pathogens
It has been known that extracellular polysaccharides are involved in virulence, immune response, and drug resistance of pathogenic bacteria. Escherichia coli K1 and Streptococcus suis are baterial pathogens causing bacteremia and meningitis in human neonat. Previously, we found that NeuA, the key enzyme responsible for the synthesis of capsular polysaccharides in E. coli K1 and S. suis, is a bi-funtional enzyme, which possesses both CMP-NeuAc sythetase and O-acetylhydrolase activities. Further investigations reveal that NeuA is a part of the regulatory mechanism to modulate the O-acetylation degree of capsule. Although the detailed mechanism remains unclear, it has been demonstrated that O-acetylation of capsule is a strategy for bacterial cells to adapt host environment. High O-acetylation of capsule enables bacteria escape from Siglect-mediated innate immunity. The knowledge obtained from our research will provide new strategy and targets for anti-meninggitis theurapies.
In addition, we have also investigated the synthetic pathway of extacellular polysaccharides in S. pneumonae. Several genes have been identified as determinators of different serotypes.
3. Expression of glycoproteins in fungi
Trichoderma reesei is a filamentous fungus identified as GRAS (generally recognized as safe) status by FDA (U.S. Food and Drug Administration). T. reesei is able to perform many post-translational modifications and grows faster than plant, insect or mammalian cells, therefore it has been thought as an attractive expression host for glycoproteins. However, the expression of heterologous protein is less efficient in T. reesei. Using genetic, biochemical and glycoproteomic approaches, we have found that O-glycosylation is involved in protein folding and cell wall synthesis. Interferring O-glycosylation can enhance expression and secretion of glycoproteins in T. reesei. Therefore, re-construction of O-glycosylation pathway could be a new strategy to improve the expression of glycoproteins in T. reesei.
In attempt to obtain an expression system for humanized glycoproteins, more recently we are also working on re-construction of glycosylation pathways in Pichia yeast.
4. Glycosylation and its function in halophilic archaeaon Haloarcula hispanica
Like eukaryotes and bacteria, archaea can decorate proteins with both N- and O-linked glycans. The most widely studied glycoprotein in archaea is the surface layer (S-layer) glycoprotein. However, our knowledge of glycosylation in archaea is still limited. Investigation of glycosylation in archaea will help to understand the function of glycosylation from the view of evolution and provide tool glyco-enzymes for Synthetic Biology. In this lab, we have investigated the glycosylation pathway in halophilic archaeaon Haloarcula hispanica. The structures of N- and O-glycan have been determined. Several genes have been identified to encode enzymes responsible for glycosylation. It has been shown that glycosylation is involved in the adaptation of H. hispanic to high-salt environment. Importantly, we discover a novel flippase (Figure 2).
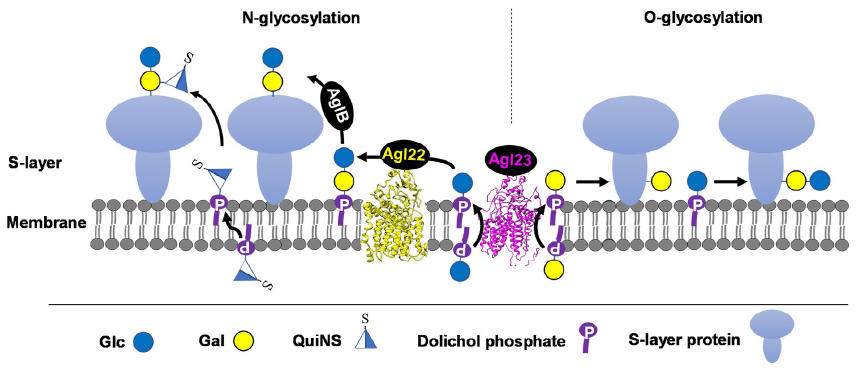
Figure 2. Schematic depiction of the N- and O-glycosylation pathways in Haloarcula hispanica. The S-layer glycoproteins of H. hispancia are modified by an N-linked Glcα-(1, 2)-[QuiNSβ-(1, 6)-]Gal trisaccharide and an O-linked Glcα-(1, 4)-Gal disaccharide. N- and O-glycosylation of the S-layer glycoproteins occur on the outer surface of the cell membrane, using DolP-linked sugars as donors. Glc-DolP and Gal-DolP are first synthesized on the cytosolic side of the cell membrane, likely using UDP-Glc and UDP-Gal as donors, respectively, via the actions of unknown glycosyltransferases. Then, Glc-DolP and Gal-DolP are flipped to the outer surface of the cell membrane in a reaction thought to involve Agl23 for subsequent use in N- and O-glycosylation. Although both N- and O-linked glycans contain Glc-Gal disaccharide part, distinct glycosidic linkages indicate different routes of their biosynthesis. The N-linked glycan is first assembled as Glcα-(1, 2)-Gal-DolP by Agl22 and transferred to N-glycosylation sites in target proteins, presumably by the oligosaccharyltransferase AglB. The third sugar, QuiNS, is then added to N-linked disaccharide by an unknown glycosyltransferase using QuiNS-DolP as a donor. The O-linked Glcα-(1, 4)-Gal disaacharide is sequentially added to O-glycosylation sites by unknown glycosyltransferases using Gal-DolP and Glc-DolP as donors.
