
Prof. Dr. Lichun He
Wuhan Institute of Physics and Mathematics, CAS
Innovation Academy for Precision Measurement Science and Technology, CAS
University of Chinese Academy of Sciences
Email: helichun@apm.ac.cn
Lichun He was appointed as professor at the Wuhan Institute of Physics and Mathematics of the Chinese Academy of Sciences in 2019. His current research focuses on understanding structure, dynamics and function of biological systems by using nuclear magnetic resonance spectroscopy and other biophysical methods. He is also interested in NMR methods development.
Research Areas
Research
1. Mechanism of Biologcial Molecules Explored via NMR spectroscopy in Solution and Its Native Enviorments
Chaperones are crucial for cellular life to ensure all proteins obtain their right fold and functionality. The promiscuous property of chaperones enables them to bind with a wide spectrum of client proteins from nascent proteins to quasi-native and native proteins. While mechanisms of chaperones aided protein folding have been intensively characterized, it is still largely unknown how chaperones act on folded clients and modulate the activity of the folded protein. To address this fundamental question, We apply NMR spectroscopy and other biophysics approaches to understand chaperone and client proteins recognition and interaction by investigating their intermediate conformations (excited state), protein dynamics modulations and the subsequently changes of its activities.


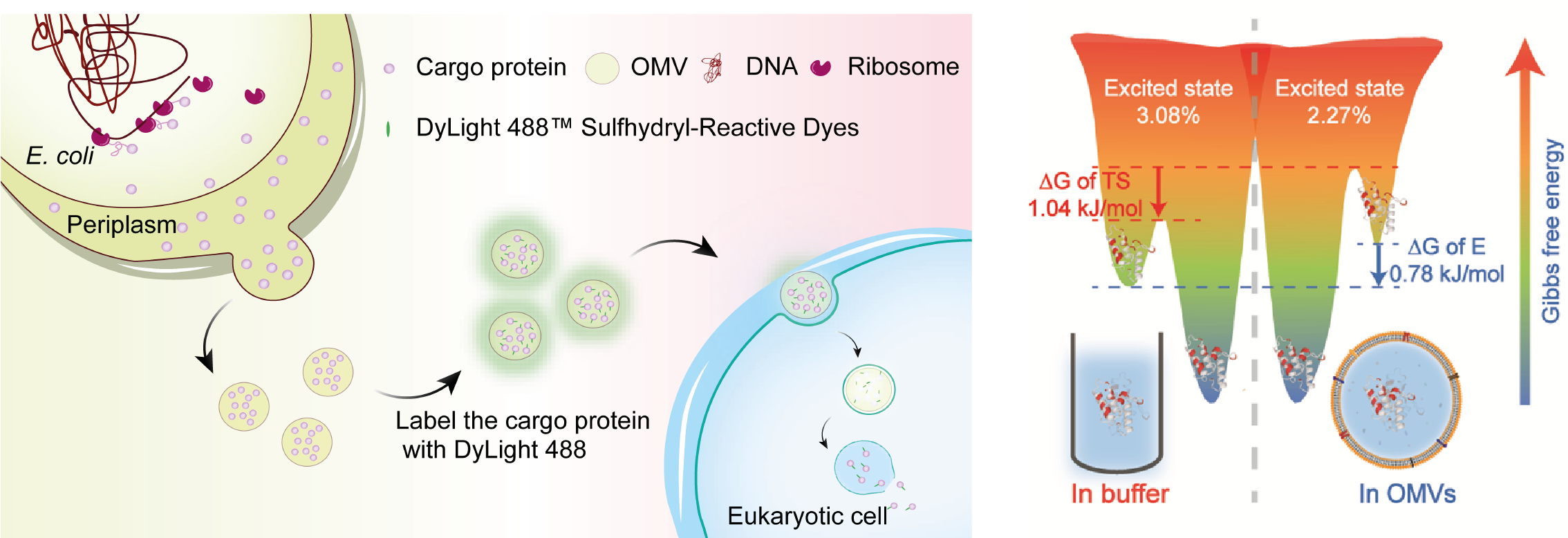
2. Mechanism of Bacteria Alter Host Innate Immune Response via Outer Membrane Vesicles(OMVs)
Bacterial outer membrane vesicles (OMVs) are nano‐sized compartments consisting of a lipid bilayer that encapsulates periplasm‐derived, luminal content. OMVs, which pinch off of Gram‐negative bacteria, are now recognized as a generalized secretion pathway which provides a means to transfer cargo to other bacterial cells as well as eukaryotic cells. Although it is well recognized that OMVs can enter and release cargo inside host cells during infection, the mechanisms of host association and uptake are not well understood. We apply biophysical, biochemical and cellular biological approaches to investigate characters of virulence factors in OMVs and study the mechanism of host cell association, uptake and innate immunue response to OMVs.

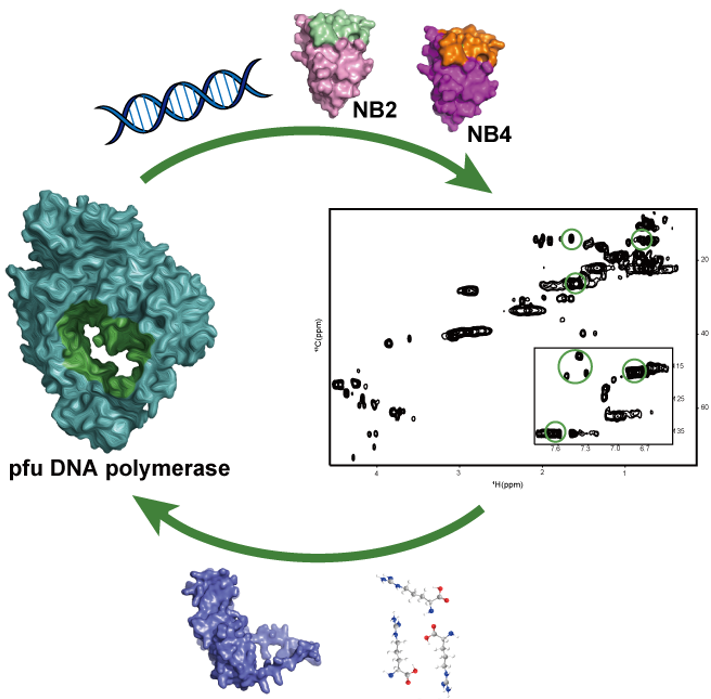
3. Nanobody Screening and Epitope Mapping via NMR
Single-domain antibody, also known as a nanobody, is an antibody fragment from Camelid consisting of a single monomeric variable antibody domain. It has a molecular weight of only 12–15 kDa. Nevertheless it is able to bind with a particular antigen with a high affinity. Besides higher affinity, smaller size, nanobody also has other advantages such as higher stability and easy expression in E.coli facilitating isotope labeling and epitope mapping via NMR.

4. NMR Directed Enzyme & Protein Evolution
Directed evolution offers a valuable method to enhance existing characteristics and introduce entirely new functionalities to protein. However, the potential of this approach is naturally limited to small proteins due to the vast number of possible amino acid sequences. To explore the complete sequence space of a protein larger than 100 amino acids, one would need to test an overwhelming 20e100 combinations, surpassing the capabilities of current experimental methods. Nevertheless, functional hot spots in enzymes can be identified using NMR spectroscopy, offering simplicity in improving the activity of enzymes. As it does not require any prior knowledge of the protein's structure or bioinformatics. We anticipate its wide applicability and its ability to unlock the full potential of directed enzyme evolution.
EDITORSHIP
Editorship & Membership
2021-09, Cell Press: The Innovation, Youth Editor
2021-07, Life, Special Issue Editor
2021-08, Biomolecules, Topical Advisory Panel Membership
Working Staff




Bin Yu Yun Peng Mengyi Wu Yaling Zhao
Postdoc Postdoc Technician Technician
Collaboration
University of Basel, Switzerland
Friedrich-Loeffler-Institut, Germany
Graduate Student






Guan Wang Yihao Chen Shixing Qi Zhiqing Tao Mingjun Zhu Ruishen Ding
Graudate Student Graudate Student Graudate Student Graudate Student Graudate Student Graudate Student


ChaoZhe Wang Huan Wang
Graudate Student Graudate Student
Undergraduate Student

Junli Long
Undergraduate
Publication
(1). Expression, purification, refolding, and characterization of octreotide-interleukin-2: a chimeric tumor-targeting protein, Int J Mol Med., 2011
(2). Solid-state NMR resonance assignments of the filament-forming CARD domain of the innate immunity signaling protein MAVS, Biomol NMR Assign., 2014
(3). Structure determination of helical filaments by solid-state NMR spectroscopy, Proc Natl Acad Sci U S A., 2016
(4). A molecular mechanism of chaperone-client recognition., Science Advances, 2016
(5). Therapeutic silence of pleiotrophin by targeted delivery of siRNA and its effect on the inhibition of tumor growth and metastasis, PLoS One, 2017
(6). An unexpected protective role of low affinity allergen-specific IgG via the inhibitory receptor FcrRIIb, J. Allergy Clin. Immunol., 2017
(7). Common Patterns in Chaperone Interactions with a Native Client Protein., Angew.Chemie Int. Ed., 2018
(8). Frustrated Interfaces Facilitate Dynamic Interactions between Native Client Proteins and Holdase Chaperones, ChembioChem, 2019
From 2019
(1). Mechanisms of Chaperones as Active Assistant/Protector for Proteins: Insights from NMR Studies, Chinese Journal of Chemistry, 2019-12
(2). NMR-Based Methods for Protein Analysis, Anal Chem., 2020
(3). Cellular stress promotes NOD1/2-dependent inflammation via the endogenous metabolite sphingosine-1-phosphate, The EMBO Journal, 2021
(4). Chaperone Spy Protects Outer Membrane Proteins from Folding Stress via Dynamic Complex Formation, mBio, 2021
(5). Backbone resonance assignments and dynamics of S. cerevisiae SERF, Biomolecular NMR Assignments, 2022
(6). Molecular Insight into the Extracellular Chaperone Serum Albumin in Modifying the Folding Free Energy Landscape of Client Proteins, Journal of Physical Chemistry Letters, 2022
(7). Self-Assembled Oligopeptide (FK)4 as a Chiral Alignment Medium for the Anisotropic NMR Analysis of Organic Compounds,ACS Applied Materials & Interfaces, 2022
(8). Diverse Roles of ScSERF in Modifying the Fibril Growth of Amyloidogenic Proteins. Chemistry, 26;29(30): e202203965, 2023
(9). Protein Conformational Exchanges Modulated by the Environment of Outer Membrane Vesicles,J Phys Chem Lett. 14 (11):2772-2777, 2023
(10). Enrichment and delivery of target proteins into the cell cytosol via Outer Membrane Vesicles. ACS Applied Materials & Interfaces. 2023
Contacts
Contact:
Prof. Dr. Lichun He
Wuhan Institute of Physics and Mathematics, CAS
Add:West No.30 Xiao Hong Shan,Wuhan 430071 China
Tel:+86-27-87197309
Email: helichun@apm.ac.cn
Join Us:
We are looking for highly motivated International Ph.D Student and Postdoc. Please contact helichun@apm.ac.cn
